TITANIUM DRY-ANODISING PROCESS: A case of success of the DRYCOAT project
Dr. Pablo Santamaría, CIDETEC Surface Engineering.
At the end of last year, the DRYCOAT project (DRY electrolytes for mass application of COATings) came to an end after almost three years of intensive and innovative research that has led to a considerable breakthrough in the development of dry electrolyte technology.
With an increasing focus on sustainable practices, with finishing methods that minimise environmental impact and use cleaner technologies, the main objective of DRYCOAT project has been to develop safe and sustainable electrochemical surface treatment processes based on dry electrolytes. The project has pursued to replace the use of traditional liquid electrolytes by dry ones and substitute the currently used baths and lines containing polluting and hazardous liquid electrolytes by a technology which can be easily integrated with the other production systems.
Dry electrolytes consist of porous polymeric particles in which the liquid electrolyte is retained without being able to escape. When the electrical current is applied to perform the electrochemical process, the particles act as an electronic bridge and allow the electrochemical reaction on the metallic surface in contact with the particles. Compared to conventional liquid surface electrochemical treatments, dry electrolyte based processes offer several advantages including: (1) environmental safety (the absence of liquid electrolytes minimizes hazardous waste generation); (2) enhanced surface quality, due to the fact that the generated layer is uniform; (3) precise control of the dry electrolyte based processes, such as voltage, current density, and electrolyte composition; (4) energy efficiency, since this technology requires low voltages and can be more energy-efficient than conventional liquid processes, and (5) material versatility, because this new process can be tailored for different metals and alloys.
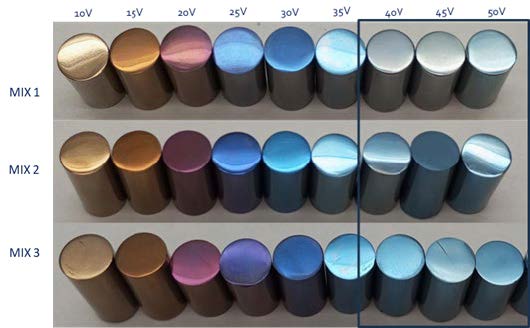
One of the studied cases within the DRYCOAT project has been titanium dry anodising process, as it is widely used in a wide range of relevant applications as aerospace, automotive and biomedical industries. In addition to titanium’s low density and high strength to weight ratio, the produced anodic oxide layer provides an improved wear and corrosion resistance as well as the possibility to obtain coloured surfaces due to an interference phenomenon. All these features provided by the anodising process add value to titanium extending its performance and enabling its use to further applications. In this scenario, one of the main efforts within the DRYCOAT project has been devoted to the understanding and optimisation of this first time explored titanium dry anodising process, with the aim of meeting the performances obtained using liquid-based routes. The study has allowed gaining a deeper understanding of the process in terms of electrical parameters and acid concentration, and the obtained results represent a huge leap forward in titanium anodising treatment effectiveness.
The results of the study, that have been recently published,[1] have proven that the developed dry electrolytes have the capacity to effectively anodise titanium, allowing the finishing colour modulation depending on the applied electrical parameters. The developed processes have been continuously monitored, and it has been found that independently of the acid concentration present in the dry electrolyte, there is a voltage limit at approximately 50-60 V, from which the excessive local heating produced by the Joule effect causes a substantial temperature increase that leads to the thermal degradation of the dry electrolyte. Although this voltage limitation restricts the available colour range that can be generated comparing to the traditional anodising process with liquid electrolytes, by the application of low-frequency pulses the possibility to reach 150V and broadens the colour range has been satisfactorily achieved. This is because the low-frequency pulses allow a better heat dissipation during the process, and this is reflected in a lower temperature increase that keeps the dry electrolyte temperature stable under service-like working conditions. Using this approach, it has been also possible to preserve the effectiveness of the media and increase the lifetime of the dry electrolyte under service-like working conditions. Regarding the thickness of the anodic layer, that has been measured using both reflectometric methods as well as advanced focussed ion beam (FIB) characterization techniques, it has been found that the thickness of the anodised layer is dependent on voltage and current density, being able to grow linearly and potentially, respectively, reaching a homogeneous layer thickness of around 60 nm. It has been also found that the thickness is not dependent on the acid content, for both investigated concentrations, as the same thickness is achieved for both dry electrolytes under the same conditions. Other relevant aspect of the study is that an iterative process has been applied to develop a predictive simulation tool for calculating the anodic layer thickness as an output parameter. This has allowed the development of a Genetic Aggregation Model that can estimate the layer thickness without the need of experimental measurement by knowing the applied voltage and anodising time. Finally, preliminary trials on dental prosthetics have been conducted with very promising results in terms of corrosion performance (open circuit potential and potentiodynamic analysis) in conditions simulating in-vivo conditions.
The DRYCOAT project has been funded in the framework of the Eurostars-Eureka research and innovation program. The project has been leaded by GPAInnova (Spain) in collaboration with 2 partners: DLyte (Spain) and Bionic Surface Technologies (Austria). Both Spanish companies have counted with the collaboration of CIDETEC Surface Engineering (Spain).
Figure 1. DRYCOAT project consortium members on the closure meeting held at CIDETEC Surface engineering facilities (left), colour range obtained for the tested dry-electrolytes containing different acid concentrations (right), and anodic layer thickness obtained as a function of the applied voltage for the tested dry electrolytes (down).
- 1. A. Valencia-Cadena; M.B. García-Blanco; B. Reschenhofer; C. Barreneche; P. Skerbis; P.A. Leitl; P. Santamaría; J.J. Roa. In-depth study of the dry-anodizing process on Ti6Al4V alloys: Effect of the acid content and electrical parameters. Surface and Coatings Technology 2025, 499, 131767









